Welcome to a page to emphasize the progress I made in physical theory. Here is a photograph of models of nuclei at a scale of 2 trillion. White protons and black neutrons are used. Carbon is shown to have two planes of protons. The theory proposes that the shape of a nucleus drives the positions of electrons ( e– not shown). The converse is true: during fusion in a star, the electrons drive protons to make lines of protons. This involves electron-proton pairing, with temporary loyalty.

Figure 1: White protons, black neutrons. Some elements magnified 5 trillion times, with iodine for biology
After stars make lines of protons, rings are made from the lines. The proton rings in the iron nucleus have a memory field of magnetic flux density B, with hysteresis. Proton rings in biologically active iodine facilitate memory spin changes in neutrons. That is easy because , theoretically, electrons pass through nuclei more often than I had expected. Tunneling may sometimes be related to the easy way for electrons to pass through nuclei that have rings. Life may be about that easy way to overcome entropy. Memory could have evolved before plant DNA existed. August 26, 2025
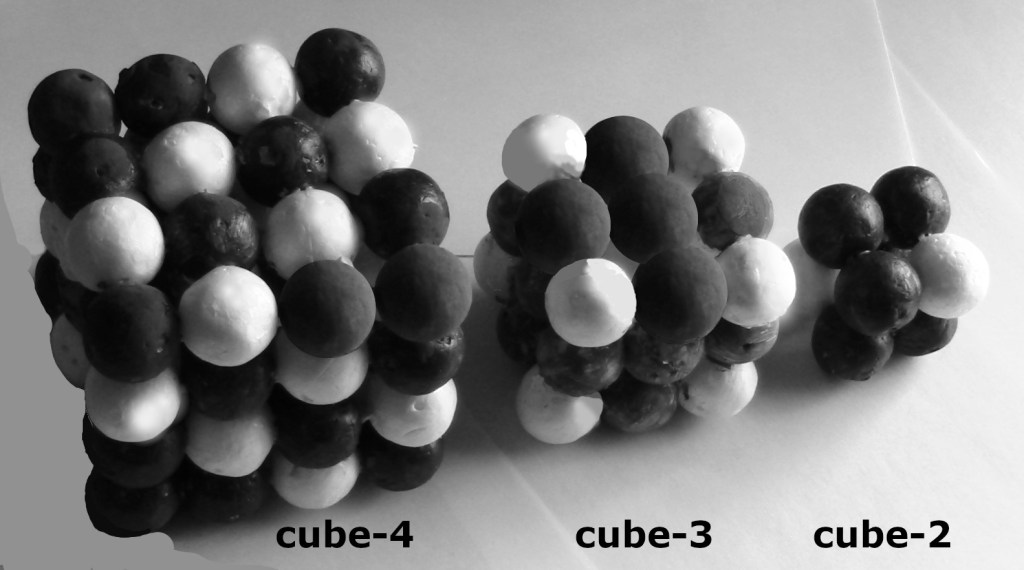
Figure 2: The insides of the promethium, iron, and nitrogen nuclei are cubic
Cubes for cores of chemical elements, white proton, dark neutron. By having a simple cubic lattice in the core, the outer surface of most nuclei have the hexagonal close-pack structure. That is the best armor to help an isotope to survive into eternity. The abundances of elements and decay rates are affected by the porousness of the surfaces of the nuclei. Iron and silicon have smooth nuclei, and they are abundant. The next figure shows what provides armor for the six faces of a cube.

Figure 3: Pyramids of baryons (protons and neutrons) to armor the six faces of each cube. Top left to right: pyramid-2, pyramid-3, pyramid-4. Bottom row: base of pyramid-6, -5, -4.
This theory of nuclear structure is based on iron. On May 25, 2017, I realized that iron-57 has a cube with six pyramids covering the six faces of the cube.
57 = 3x3x3 + 6*5 protons and neutrons
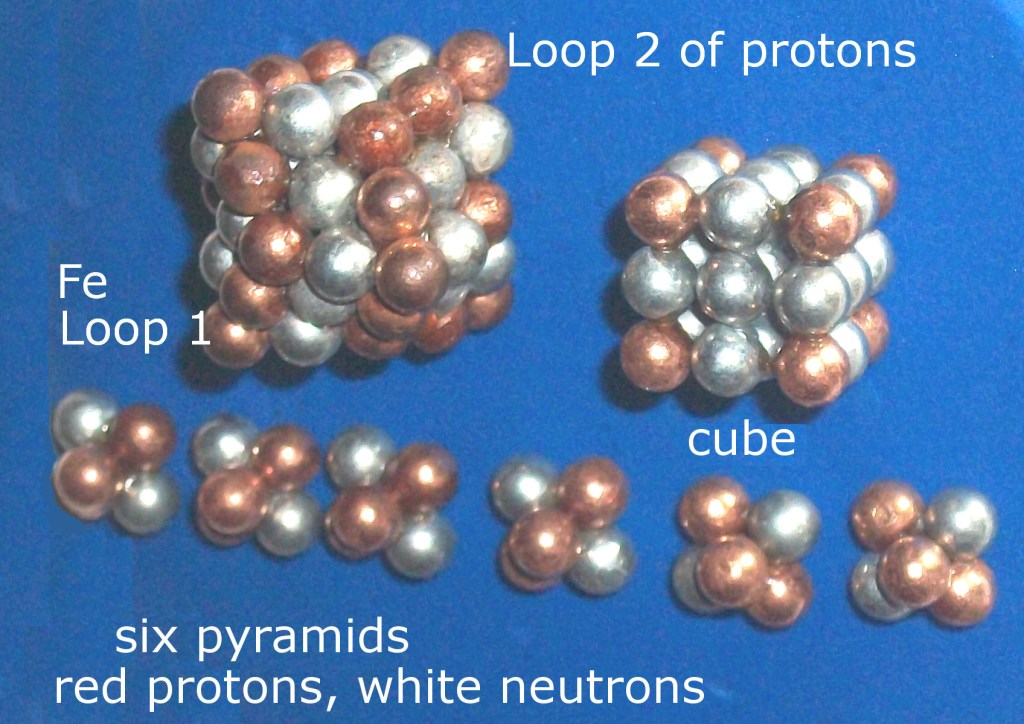
Figure 4: orange protons, gray neutrons
Iron’s theoretical nuclear discovery lead to all other elements’ structures being proposed to follow the same Rules. Photo from May, 2018 in Maui.
The protons and neutrons are numbered for each nucleus. The book shows the numbers on Figures and also, this website has this link to enlarged Figures from the book. A paper about iron was published in March, 2019 in the Journal of Nuclear Physics.
Simplified Flux Inside the Iron Nucleus and Outside
Here is an image of the simplified mock-up and the precision mock-up that will be used in a video. This will show how proton rings use the geometry of the axial proton to explain what the algebra of energy cannot.
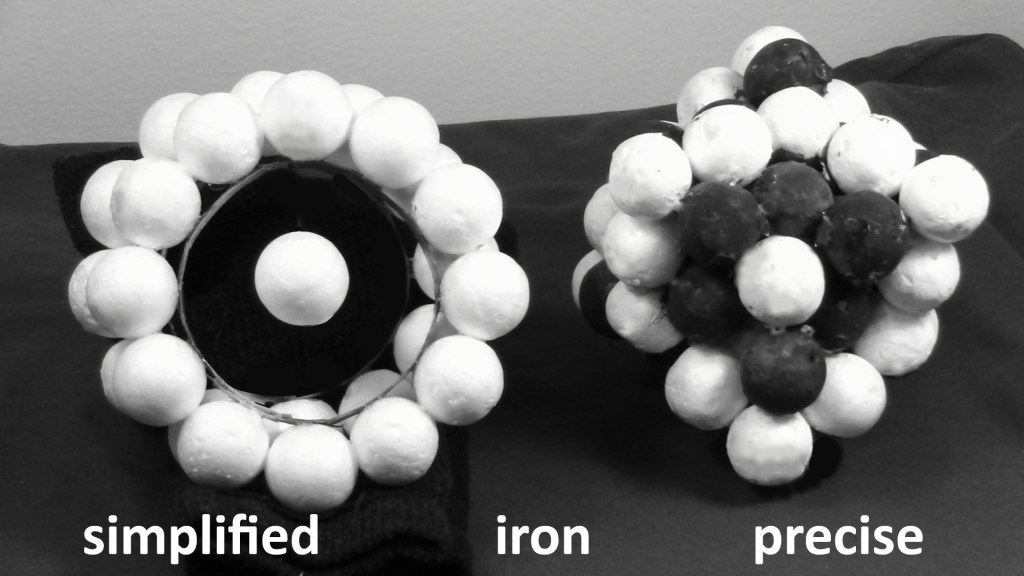
Figure 5: the lone proton is called the axial proton because it on the axis of two rings. The simplified model has no neutrons visible.
The following figures show steps in the construction of the model of the interior flux in iron. All 26 protons are on the surface of the nucleus. There are twelve in each ring plus two axial protons on the surface of the nucleus. Iron is the ideal ferromagnetic element. Neodymium is nonferrous because it does not have axial protons on the surface of the nucleus. Gadolinium is ferromagnetic below room temperature because it has two axial protons on each end of the nuclear surface. At cryogenic temperatures, other rare-earth elements also are ferromagnetic. Paramagnetic elements like oxygen, can never be ferromagnetic if they do not have two coaxial rings of protons. Magnesium has one ring of twelve protons. Chlorine has a ring of 8 protons.
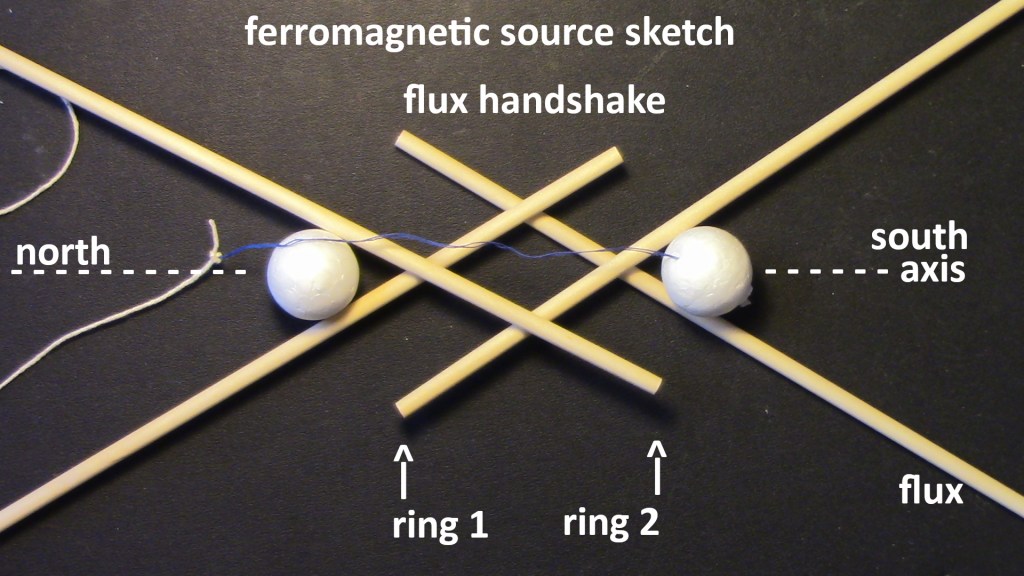
Figure 6: Simplified interior of iron nucleus, ring 1 makes south pole. The two white protons are the “axial protons” that ferromagnetic elements have. They limit the angles of the divergence of mechanistic magnetic lines of flux (yellow sticks). The axial proton has a spin that is compatible with the ring current direction. Nd does not have an axial proton on the surface of the nucleus, so it is nonferrous. The string is not used.

Figure 7: compare this precise model with Fig. 6 simplification of iron
The proton rings in Fig. 7 have the undulator shape. That slows down the hysteresis phenomenon by introducing a variety of types of handshaking encounters between magnetic lines of flux. This is not about energy. Algebra was the wrong tool to try to describe ferromagnetism. Flux is the wavefunction.
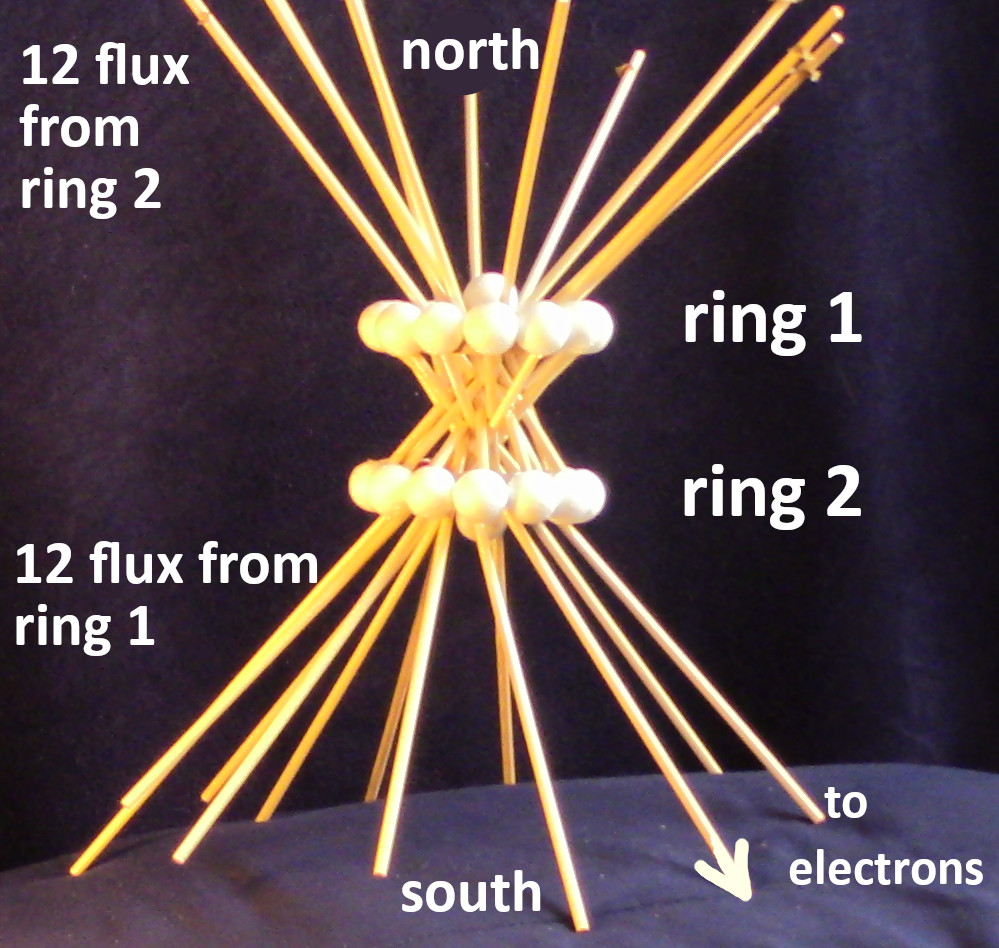
Figure 8: completed iron mock-up by Alan C. Folmsbee, February 12, 2025. The axial protons are shown.
Figure 9: Lab scene during preparations to show Flux Spin Handshaking Ferromagnetic Theory
The Flux Handshaking Ferromagnetic Theory uses two pseudo-monopoles so one proton ring makes a South Magnetic Pole and the other proton ring makes a North Magnetic Pole! Monopoles are not possible. This is different from an electromagnetic coil of copper wires. An electromagnet makes a north pole and a south pole for any one loop of wire. Iron makes a north pole (Fig. 8) using ring 2 and a south pole from ring 1 only due to the flux vortexes touching each other and their spins being coordinated at the center of the nucleus. Those two vortexes touching inside the cube are determining a right-located electron eddy, or a left-located electron eddy, relative to proton rings. Opposite spins begin to bond, same spins slide. A copper solenoid electromagnet produces both eddies, far from each other, without a flux handshake to influence their positions.
Even if an apparatus is made with one pseudo-monopole, the observer can look at the pole from two remote positions to conclude that it has a south and a north pole. The observer only moves and rotates the head to change a north pole to a south pole. The magnet does not change. An apparatus could use magnesium because its nucleus has one ring with twelve protons.
Poles are made by rotational motion, so a monopole cannot exist. Old science has faith in S algebra to make a magnet. Spin S was a placeholder, a symbol that was empty.
The point is that all motion in an iron atom can be clockwise (for example): proton currents in both rings can be clockwise while electron eddy motions of north and south can be clockwise, to a fixed observer. But the surprise (Fig. 6) is the polarization of right-pointing flux lines for ring 1 and left-pointing flux lines for ring 2. I propose the theory of handshaking to negotiate that specialization. Spins of electrons are connected by spins of flux to influence spins of protons. Two flux lines can only slide by each other if the spins are correct. If the wrong flux spins are impacting, they start to bind but fail, seeking employment elsewhere. That must occur when the left and right directions make north and south. Inside the cube of a nucleus, the small volume forces lines to meet lines, unlike in a solenoid coil of wires. The proton ring has a superconducting loyalty current. That current directly causes electron spin current. A pair has a direct interaction from a proton to an electron and back. Each pair induces adjacent pairs to react. A third video is on the page of Papers.
Videos of the Rotating Iron and Flux Model
The confluence zone shows how iron magnets have torque, leverage, and a fulcrum from which to move remote sub-universes (in molecules). The protons would be stationary and the flux moving for a real nucleus. A “superconducting loyalty current” flows in each of the proton rings for an iron atom that is magnetized. Electrons would be at the ends of the sticks, moving with the same rotation direction as the proton current. Lenz’s Law is followed.
Fig. 9a is a simplified iron nucleus with internal flux nexus. White protons make 24 lines of yellow flux, each from a proton to an electron. The model is inaccurate, as the protons are moving. Instead, imagine the revolving white balls represent proton current, not proton motion. Superconducting proton current flows in the rings of magnetized iron. That is called the “loyalty current”. Each electron shares loyalty with only one proton, temporarily. In theory. ACF November 25, 2025.
Coercivity Theory
Now that the interiors of magnetic nuclei are known, the high coercivity of NdFeB magnets is explained. In neodymium, the cube in the core has 14 protons. Some of those constrain the changes in flux directions. That prevents the easy changes in north/south polarity that are observed for iron. A “pair” is an electron and its loyal proton.
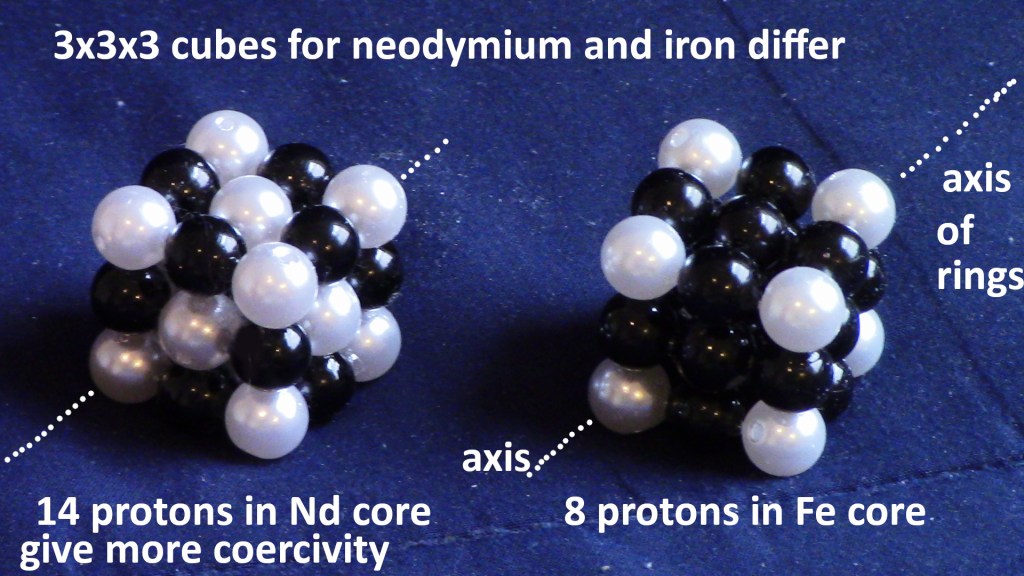
Figure 10: cubes inside Nd and Fe are 3x3x3, but they differ. Nd has all of those cube protons buried in the interior of the nucleus. Fe has all of the cube protons on its surface. Therefore Nd is nonferrous and Fe is ferromagnetic.
Iron nucleus with no neutrons visible. Also see Fig. 11 of iron Blender simulation. Flux vortexes, north and south, slowly negotiate passage. Imagine 8 more protons in the center, like neodymium (Fig. 12). They make it more difficult to move fluxes to remote locations, 9fm away from the core. Then reversal from south to north takes place with 8 added protons constraining the handshake to be more difficult. That means the coercivity is greater for Nd than Fe. Therefore, the applied H-field that is used to swap poles must be a higher intensity to reduce the atom’s B-field output to zero. That is higher coercivity, which is a desirable property for permanent magnets, so they do not easily lose their magnetization under dynamic load conditions.
In Fig. 10b is a simplified iron nucleus with internal flux nexus. White protons make 24 lines of yellow flux, each from a proton to an electron. The model is inaccurate, as the protons are moving. Instead, imagine the revolving white balls represent proton current, not proton motion. Superconducting proton current flows in the rings of magnetized iron. That is called the “loyalty current”. Each electron shares loyalty with only one proton, temporarily. In theory. ACF November 25, 2025.

Figure 11: iron showing the core is empty of protons. Proton number 1 is on the surface of the nucleus, on the axis. The lack of interior protons gives low coercivity. One ring is highlighted with 12 yellow dots. There are 8 protons in the cube: 6 in the rings and 2 on the axis.
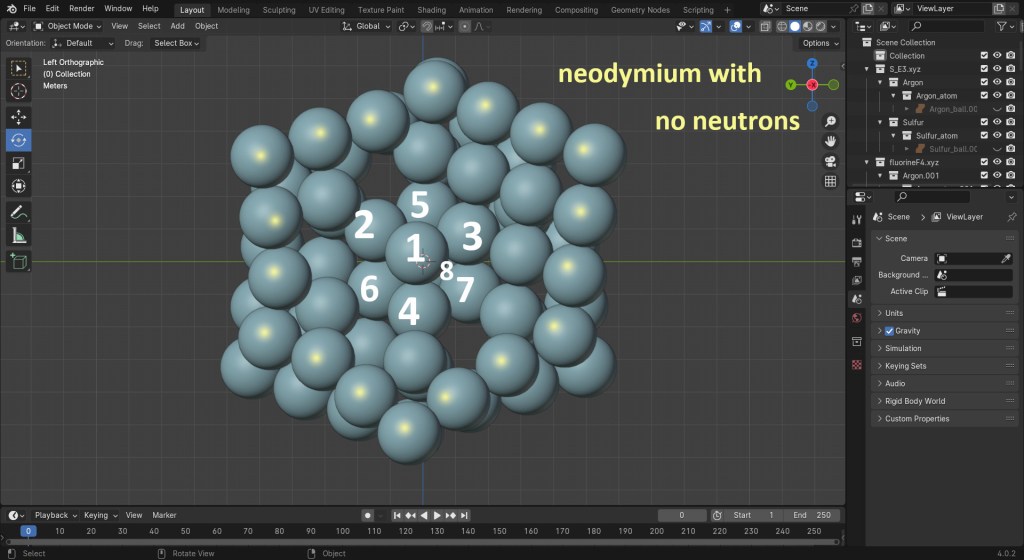
Figure 12: neodymium with labels on 8 protons in the center of the core, causing high coercivity. The cube has 14 protons but none are on the surface of the nucleus and none are in the rings. One ring is highlighted with 18 yellow dots.
Memory in animal brains
This human brain theory has the same physics of iodine as my theory of the origin of life. Before RNA biology, iodine neutron spins were used to record something about a successful cell. The memoglobin molecule came before plants or cyanobacteria. That most-primitive memory molecule was almost unchanged for billions of years. A neuron has millions of iodine atoms and a thousand of those are in a thousand memoglobin molecules. The neuron uses those thousand qubits for a main memory, plus context memory, and perspective directional memory. The iodine atom does not have a chemical reaction to read or write a qubit. Iodine is the heaviest element in life. Z=53 protons. See paper 17 of October, 2025.
Precision of this sphere-stacking theory
This is a precise theory. The Static Nucleus Theory of the Face-Armored Cubic Lattice is the correct theory of matter. Trust me. A prominent reason to believe in this precision comes from gadolinium. Ranking all 118 chemical elements by their similarity to iron’s nuclear shape, the result is perfect. Cobalt, nickel and gadolinium have nuclei that have “smooth” proton rings and axial protons, like iron. Other elements, like iodine, have proton rings with discontinuities that point into the core. See paper 10 here. That paper on hysteresis has details, like the still image in Fig. 5, above. That link also leads to Video 3 of 3.
Nuclear Binding Energy Graph Slopes
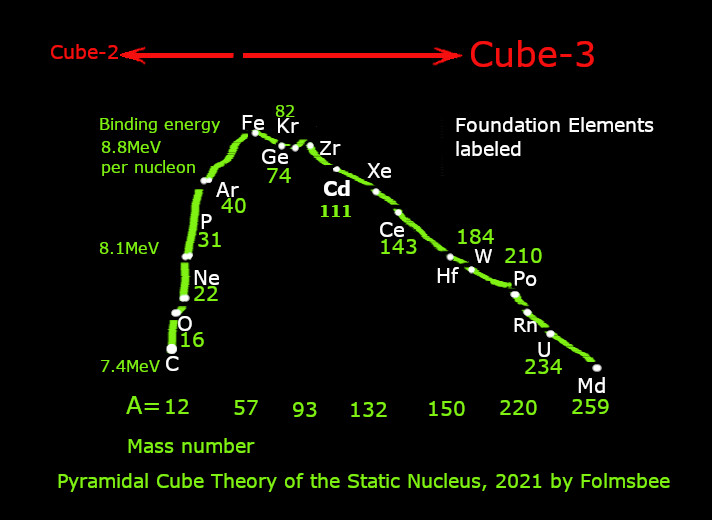
The precision of the sphere-stacking theory is amazing when looking at a graph of average binding energy per nucleon versus A, mass number. The foundation elements occur at points where the curve slope changes (link Fig 2a near paper #13). The slope is positive below Fe and negative above Fe on the graph. The encyclopedia link shows in the second graph, what I write about slopes, next.
The light foundation elements are porous and heavier elements are made as protons are stacked as spheres with gaps between them. A hydrodynamic idea is used to explain why the binding energy becomes higher as nucleons are added to light elements from carbon to manganese. Treat the strong nuclear force as a fluid draining into each spherical nucleon. If a proton is added and it clogs a gap between spheres, the pressure increases in the center of the nucleus. The next nucleon will encounter that increased pressure or energy. That explains the positive slope of Fig. 2a linked above. Light elements have a 2-layer cube in the core.
Starting at iron, the heavy foundation nuclei are smooth, and adding protons and neutrons is less energetic than the magnitude of energy needed to remove a nucleon from a foundation element. So each added nucleon has a smaller binding energy than the average of all of the binding energies of all of the previous nucleons. The rules mention that. Heavy elements have a 3-layer cube in the core. Pm and Tc have 4 layers.
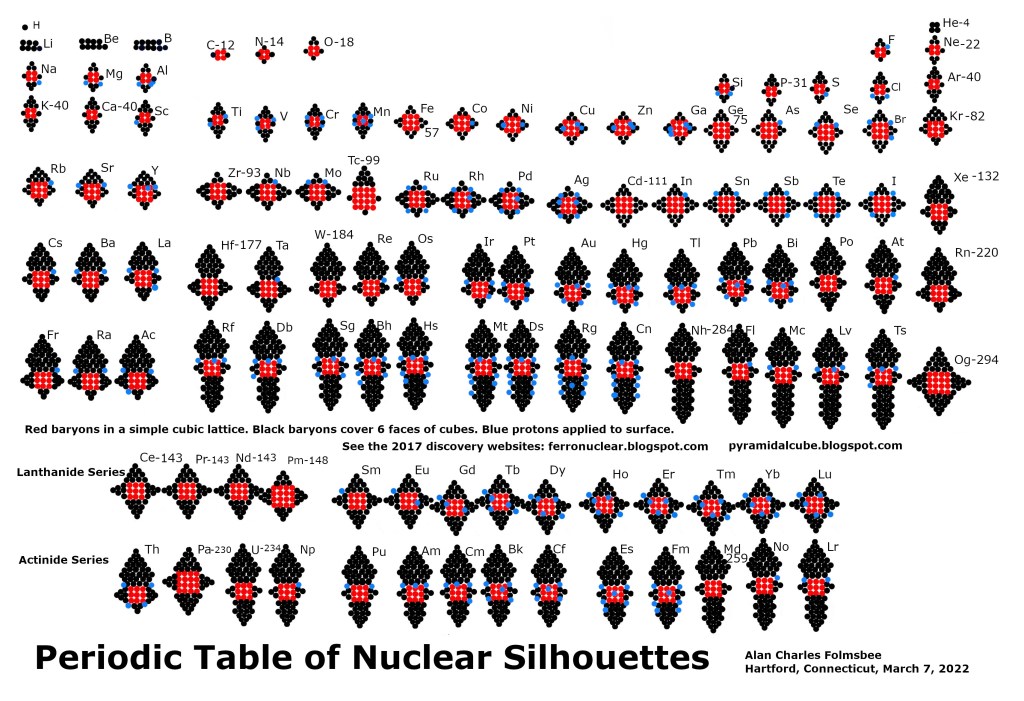
Periodic Table with 19 foundation elements C, O, Ne, P, Ar, Fe, Ge, Kr, Zr, Cd, Xe, Ce, Hf, W, Po, Rn, U, Md, Nh. Compare porous Ar with smooth Fe, Zr, Cd or Ce.
The precise water bond angle is due to the angle between proton lines of oxygen.
Another fact is that the number of proton line ends is limited to 8 and chemical valence is limited to 8. Even though element 92 has 92 protons, the number of ends of proton lines is less than 8. That is because of a new Law:
Law: A proton can touch a proton in a stable state. Three protons make a line of protons that touch, at most, two other protons.
Naturally: Lines of 8 or more protons can make rings.
Papers
Experiments were carried out on iron for more than a thousand years. A Chinese inventor made the first magnetic compass. Iron is the best choice to start understanding the nuclear structure. Fe has two rings of protons, like a magnetic transformer has two coils of copper wires. That correspondence is a dream come true. Other scientists have started with hydrogen as the first element to study. That is not a good choice. They needed the obvious properties of iron to discover a correspondence between lines of protons and properties. Form and function are the clues to iron’s model being theoretically correct. Lighter elements are not so clear, as iron is, about how the property is caused by the shapes of proton lines. Chromium is the only element with the antiferromagnetic property. I wrote a paper about that in July, 2021…

Uranium mock-up using chocolates. These models were eaten by insects in 2018 in Maui. Uranium has the hexagonal close-pack lattice visible on the big end of the nucleus. That armor drives the bi-modal mass distribution for fission fragments. The big end stays together to some degree, more than the porous end of the nucleus.
Law of physics: protons usually form lines of protons in elements
Pairing of an electron and a proton
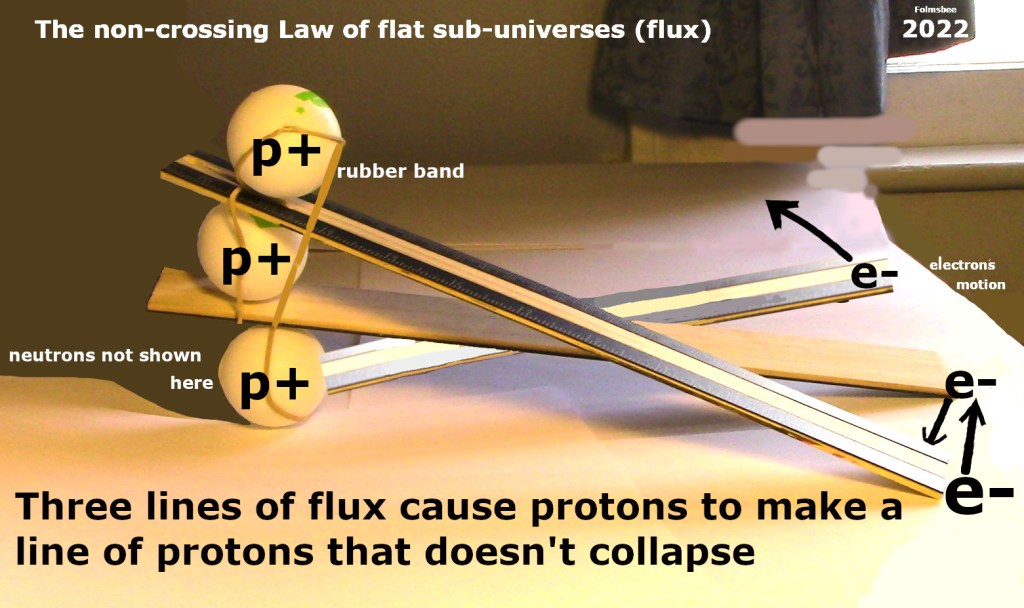
The theoretical idea is promoted about the importance of including a law of pairing. The certainty about this is due to the way iron is fused in a star. When iron is created, protons form lines of protons that join into rings of protons. That is because of two reasons. First, there are not enough neutrons to isolate the protons from each other. Second, electrons force the protons to stack up to make lines. The flat flux shape is involved (see figure with three rulers, below).
Laws of Paired Charges For Most Practical Purposes
1 There is an equal number of protons and electrons in the universe.
2 A direct interaction exists for each proton with one electron, exclusively
3 An induced interaction exists so a moving pair can induce motion in an adjacent pair.
4 A magnetic field B will make an ion gyrate clockwise when an electron gyrates counter-clockwise. That is because the line of flux has a flat, conveyor-belt action, described elsewhere.
Example: commercial voltage transformer
A primary coil has an electron flowing and it is paired with a proton in a secondary coil. The magnetic line of flux that connects the pair is pushing adjacent lines of flux. That induces motion in other charges. An opposite voltage is induced as the primary electron motion induces flux motions for the secondary coil proton-electron pairs. That is called Lenz’s Law. A local coordinate system can be tried for the various pairs to model the known gyrations and Lenz’s Law. The 4D universe uses xyz and t. The analogy is for a pair to use hx hy hz and th. The neutrons receive the hz dimension to feed them. Free neutrons decay in minutes without the hz food from protons. With no neutron, a proton exudes hz into free space, perhaps causing expansion. This might be a neutrino, a length of dimension hz. It has no area, so it could be massless.
Theory Highlights
One of the highlights is that electrons are affected by the positions of protons. Carbon has flat crystals to are called graphite. The protons in carbon are arranged as two parallel triangles. That shape drives the flat crystals that the atoms make. This nuclear theory can make chemistry become a more powerful technology.
Catalysts can be related to the nuclear structures. The hooked line of protons in platinum is expected to lead us to insights.
The proton lines in medium-sized elements can be used in simulations to provide the direction of one end of a bond. The proton line can also define where torque originates for a bond. Macroscopic functions are made by nuclear shapes. Protons can attract electrons, repel electrons and scatter electrons
The most prominent protons on a nucleus can be identified. This is believed to be a reason for valence electrons to be limited in number. Even though an atom has 40 electrons and 40 protons, only 4 valence electrons might be significant to a chemist. Those valence electrons may be associated with the prominent protons. Sulfur and carbon are examples of a valence 6 and 4 match the nuclear proton line end counts.
Experiments were carried out by other researchers and this paper is featured: “Transient ferromagnetic-like state mediating ultrafast reversal of antiferromagnetically coupled spins” published in Nature, 472 in 2011 and authored by I. Raud, K. Vahaplar, C. Stamm… et al. This uses an alloy of GdFeCo so a flash of light reverses a north pole to become south. 3 Fe atoms surround one Gd atom. (Cobalt is like iron.) I wrote a paper about that, and you can see it, next.
Experiments show protons make lines of protons in a randomized bead model. The images show random iron-57 candidates. On left are five images using 10mm white protons and 7mm black neutrons. On the right are five images with 8mm white protons and 9.5mm black neutrons.
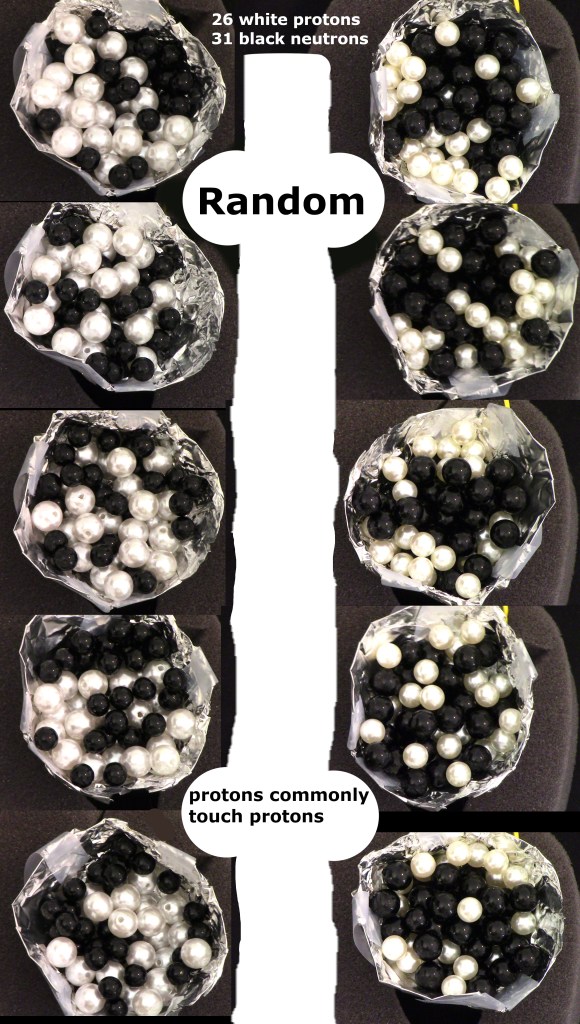

The experiment shows that protons must touch protons when in a cluster. This leads to the conclusion that a line of several protons can form. With help from electrons that have flat lines of flux, the protons are forced into non-random lines and rings of protons.
Functions of ferromagnetism come together in the iron nucleus: a ring of 12 protons, two rings, two axial protons, bends in the ring are not pointed inward to core. Some of the electrons for an iron bar magnet are known to have unpaired spins. The reason the electron spins are unpaired is because the protons touch protons and carry a current. The adjacent protons have a polarization that is coordinated with each other, so the electrons have coordinated spins: spinning the same way. Spinning the same way means unpaired. Paired means one electron with an up spin and another electron with a down spin. The protons drive electrons using the wavefunction and the opposite is true. Electrons drive proton positions during fusion and they drive proton currents during a magnetization process for an iron atom.
Each electron is always paired with a proton: it can be associated with one nucleus or dissociated, but always paired. Each electron is loyal to one proton until it switches to a different proton. Induction occurs between a paired electron-proton and other pairs.
October 19, 2024 by Alan C. Folmsbee
email omnilobe at gmail dot com
The strong nuclear force pulls each nucleus together. The average binding energy of a proton or neutron is known for all elements.
Light elements have porous shapes for the foundation elements. Starting with iron, foundation elements have smooth shapes. That is why the curve has two slopes for average binding energy per nucleon. See Encyclopedia Britannica

Iron has a 3 layer cube and argon has a 2-layer cube. That makes the argon porous. The small integers of light elements cause a coarse granularity of the discrete locations available for adding a nucleon into the lowest pit. (There are green protons and white neutrons in mock-ups).
December 3, 2024
The answer to an old question
Why are chemical valences limited to the range from 0 to 8, even though many elements have more than 60 protons?
Because a new law says that protons make lines of protons. Therefore, heavy elements have up to 4 lines of protons of various lengths, like most light elements also have less than 5 lines of protons. Apparently, the ends of lines are important for chemical bonds. Each line has 2 ends, so most elements are limited to about 8 ends of proton lines. A valence of 8 has the same number. This seems to be a trend, with the range of ends equal to the range of valences. This equality of value is independent of an energy balance. As an example, carbon has 4 ends of lines and a heavy element with 18 protons in one line and 28 protons in another line only has 4 ends.
This is supporting evidence of the correctness of the theory called The Static Nucleus Theory of the Face Armored Cubic Lattice. December 27, 2024
Hysteresis Philosophy
The magnetization of iron, and demagnetization are discussed in this paper by Mr. Ewing in 1891. How can one molecule have hysteresis? It must take two separated current loops to have hysteresis, as described by Alfred Ewing. He was quoted by Oliver Heaviside.
My theory of the iron nucleus resolves James Alfred Ewing’s quest to understand magnetic hysteresis in rotatable molecules. Maxwell proposed that many molecules act together with many loops of current. I claim that iron has two rings of protons that do not rotate relative to each other and hysteresis is caused by nuclear structures with two superconducting current loops.