The theory is named The Static Nucleus Theory of the Face-Armored Cubic Lattice. The hardcover book gives the Rules. See Charge distributions on the nuclei. The rules do not call for rings of protons. New Laws of nature are highlighted. Physical trends are also recognized, even if they are not laws.
The Nineteen Rules of Nuclear Structure Using the Static Nucleus Theory
Rule 1. Law #1:There is a simple cubic lattice of protons and neutrons at the core of each element that has a Z atomic number that is greater than five.
Rule 2: Protons in the cube are far from each other as if electrostatic repulsion is in effect in all three dimensions.
Rule 3: The six faces of the cube are armored by pyramids of protons and neutrons.
Rule 4, Law #2 Protons outside of the cube tend to form lines of protons as if electrostatic repulsion is not true in all three dimensions.
Rule 5: There are 19 foundation elements upon which the 90 incremental elements are built. The 19 foundation elements are:
carbon, oxygen, neon, phosphorus, argon, iron,
germanium, krypton, zirconium, cadmium, xenon, cerium, hafnium,
tungsten, polonium, radon, uranium, mendelevium, and nihonium.
Rule 6: The shapes of foundation elements do not depend on protons being different from neutrons. Both are treated equally, as baryons, to define the silhouettes and 3D shapes of each element.
Rule 7: Four sides of the cube have pyramids of the same shape (axial symmetry), for foundation elements. The top and bottom pyramids can have different sizes. All of the side pyramids are equal in size and shape. Rotations of pyramids do not need to be identical when positioned on the four side faces of a cube.
Rule 8: Pyramids should be rotated to Law #3: avoid creating a three-way intersection of lines of protons. Some elements cannot avoid that structure, like promethium and nitrogen.
Rule 9: Incremental elements have added nucleons on the exteriors of foundation elements to fill the gaps between pyramids. There are 90 incremental elements based on the foundation elements. Nine elements are not based on a foundation element. They are H, He, Li, Be, B, Tc, Pm, Pa, and Og.
Rule 10: An incremental element is assembled by first placing the neutrons into the deepest pits of a foundation element and then adding one proton into the deepest pit where protons tend to form lines of protons. If a line cannot be formed, the added proton can go anywhere that does not join 3 protons together in a triangle. If that is not possible, a proton can go anywhere.
Rule 11: Light elements have a sparse allocation of protons near the center and a denser allocation of protons near the tips of pyramids.
Rule 12: Pyramids can be up to six layers thick.
Rule 13: Contraction of pyramid bases occurs increasingly with heavier elements. A six-layer pyramid can rest on a five-layer contracted base, which can reside on a four-layer contracted base, which can reside in a three-layer cube, nestled into a stable arrangement.
Rule 14, Law #4: Sphere stacking for a pyramid does not need to nestle into pits of a cube and the pyramid can be stacked onto a cube vertically. For example, in oxygen, a two-layer pyramid can be stacked onto a two-layer cube.
Rule 15: Pyramids can have lines of protons plus additional protons at the corners of pyramids to achieve the Z atomic number that is known by standard science.
Rule 16, Law #5: Symmetrical arrangements of protons are preferred over non-symmetrical structures. The same is true for neutrons. The two-layer pyramid sets the example in iron. The cube-2 and cube-3 are also symmetrical in their allocations of protons and neutrons.
Rule 17: Each nucleus is shaped to provide the isotopes with A and Z which were established from old experiments for established physical tables. A is the mass number. A is equal to the number of protons plus the number of neutrons. Z is the atomic number. Z is equal to the number of protons in an element.
Rule 18, Law #6: Each proton has one electron paired with it using a flat line of flux. Flux is a mechanistic object in dynamic plasma conditions and in molecules. Several electrons’ flux lines drive multiple protons into a single line of protons that touch each other.
Rule 19: The longest distance from each neutron to a proton is one diameter of a neutron.
Video of iron-57 nucleus, white proton rings and two protons on their axis

Figure 1: Uranium-234 isotope is a foundation element for eight similar chemical elements.
You can read my paperback book on nuclear structural theory: Nuclear structure from sphere stacking. $24

Figure 2: Uranium assembly shows the cube 3x3x3 before its armor is applied.
Notes on Rules
The rules 1 and 2 use cubes with the following cube proton counts for each element
H to B, 0
C to Mn, 2
Fe to Br, 8
Kr to Ag, 9 except Tc
Cd to Ts 14 except Pm, Pa, and Og
Notice that the light elements have 2 core protons and 2 core electrons. A transition to 8 core protons happens at Fe.
All elements use the Rules from the iron research, May 25, 2017
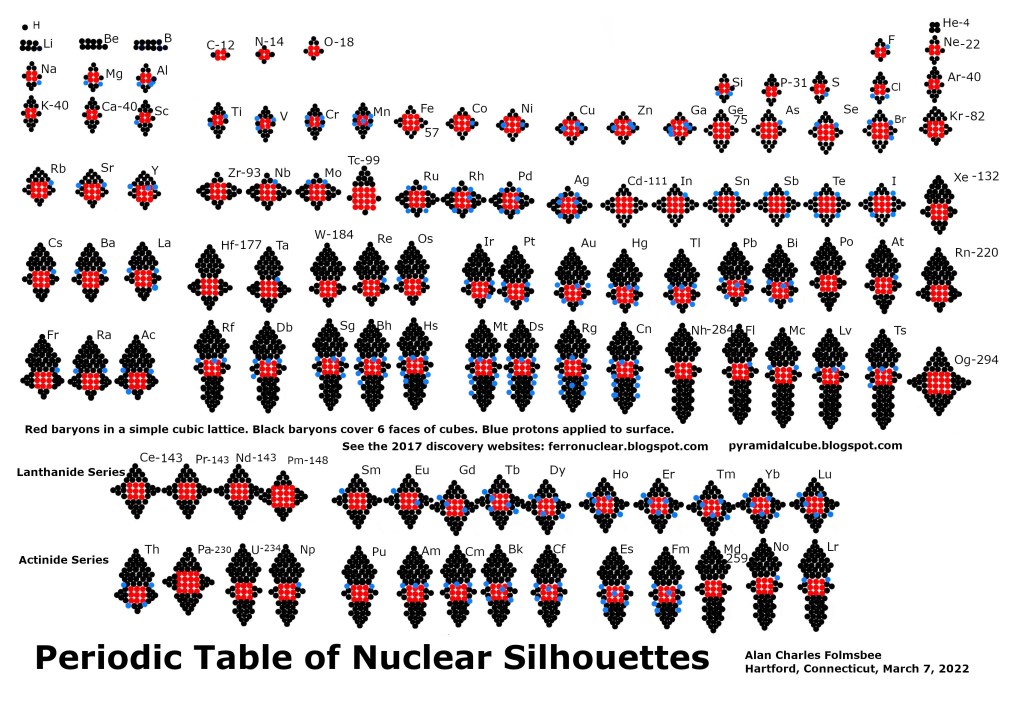
Figure 3: Schematic portrayal of plans for each element
The periodic table uses red circles for protons and neutrons (baryons) that are stacked in a cubic shape. The black circles are mostly in pyramids that cover the six faces of each cube. The blue circles are put on each incremental element to symbolize protons of elements that are slightly heavier than a foundation element. A “foundation element” has a 2 or 3 or 4-layer cube of baryons plus six pyramids of baryons that armor the six faces of the cubic lattice. The foundation element serves as a starting shape for the more-numerous “incremental elements”.
As result of the core cubic lattice, the surfaces of most elements are predominantly a hexagonal-close-pack (HCP) shape. That helps the nucleus to survive an eternity of collisions. Iron and silicon have surfaces that are completely HCP.

Figure 4: Common elements in the crust of a planet
The HCP on the surface is caused by the Simple Cubic Lattice in the core. Fe has 3x3x3 and Si has 2x2x2 for the cube sizes. The Rules are theories that are based on experiments. The nucleus of iron makes the atoms of iron show a triangular north magnetic pole under a scanning tunneling microscope. The nucleus also has a triangle of protons in a ring.

Figure 5: Iron teaches us about atomic shapes and nuclear shapes being related.
The Rules are confirmed in several ways.
Rule 4 is justified by the image at the bottom of my theory page. Protons make lines of protons, even in a random allocation of 26 protons and 31 neutrons. There are not enough neutrons to isolate the protons from each other. Also, my theory asserts that electrons drive protons to make lines of protons, even inside stars.
Go to the bottom of the page about scientific papers by Alan Folmsbee to see the 14 research decisions that resulted in the Rules (also paper #6, there). Or read the paper about my thought process.
February 12, 2025
Alan C. Folmsbee